Video of boiling point of fuel
Vapor is present in the air below the boiling point of a liquid.
Gasoline is different than water or grain alcohol in that it is a mix of many different molecules. So it has a wide range of temperatures in which components will completely vaporize. A distillation curve is used to show how much vaporizes over this range of temperatures. There are at least two industry standards for testing this. Most race fuels and av gas manufactures will supply at least 5 points on the curve. For pump fuels, the curves are harder to come by. In part this may be because there are so many different blends to address climates, regulations, and economics. However I think its because they really dont want to share.
Here's an illustration of the volatility of some gasolines.
It shows a non-reformulated gasoline from 2007, and one from that time with 8% ethanol. (That's not the same fuel).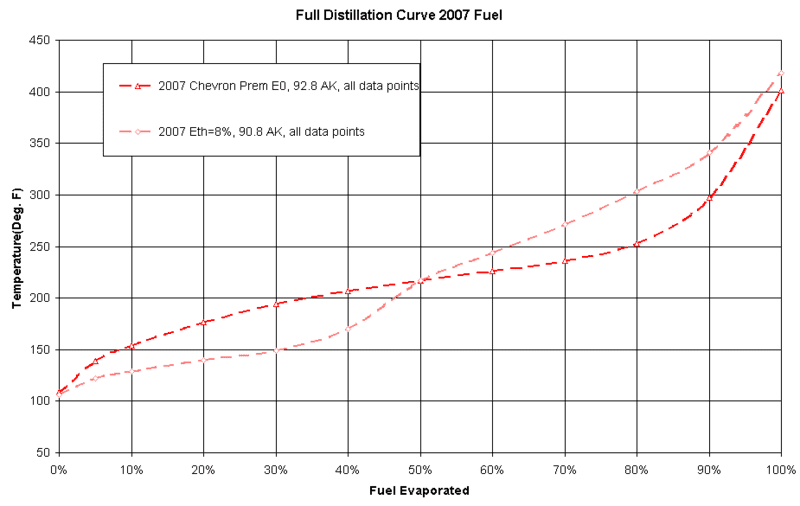
These curves show that at 150* F, around 7% of the Chevron Premium had evaporated; while the same 150* F caused 30% of the 8% ethanol fuel to evaporate!
For testing purposes there has to be standards. One of these that was widely used when studying reformulated fuel is called Tier 2 EEE. The Tier 2 EEE curve plotted here is from a tested sample used in the ACE Optimal Blend Study 2007. In other studies they use their own baseline. Shown below are distillation curves from some of the fuels from the "CRC-E67" study by Durbin, et al.
Fuel A was the baseline,
Fuel B wsa similar but with 5% eth
Fuel K was a baseline using MTBE laced fuel, 0% ethanol.
Fuel L was similar with MTBE but 10 % eth.
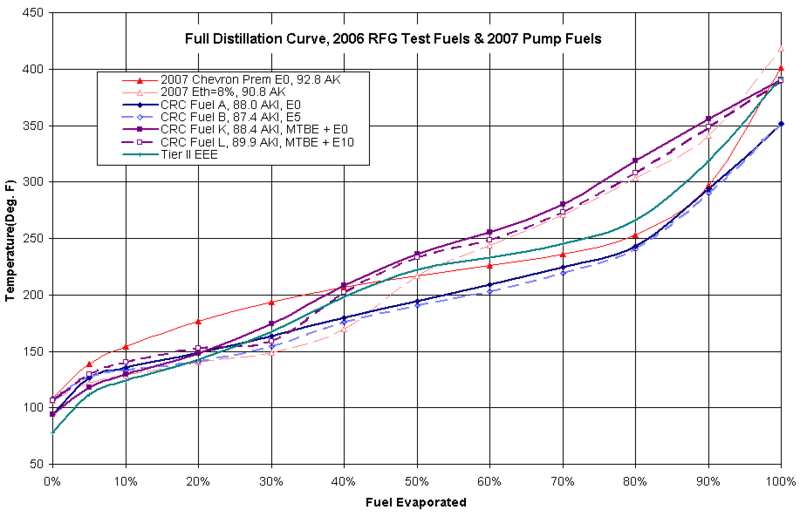
Not surprising, the fuels that evaporate more at lower temperatures tend to have a higher Reid Vapor Pressure.
RVP for:
Chevron 6.7
Fuel A 7.74
Fuel L 7.6
Tier 2 EEE 9.07
Vapor is present in the air below the boiling point of a liquid.
Gasoline is different than water or grain alcohol in that it is a mix of many different molecules. So it has a wide range of temperatures in which components will completely vaporize. A distillation curve is used to show how much vaporizes over this range of temperatures. There are at least two industry standards for testing this. Most race fuels and av gas manufactures will supply at least 5 points on the curve. For pump fuels, the curves are harder to come by. In part this may be because there are so many different blends to address climates, regulations, and economics. However I think its because they really dont want to share.
Here's an illustration of the volatility of some gasolines.
It shows a non-reformulated gasoline from 2007, and one from that time with 8% ethanol. (That's not the same fuel).
These curves show that at 150* F, around 7% of the Chevron Premium had evaporated; while the same 150* F caused 30% of the 8% ethanol fuel to evaporate!
For testing purposes there has to be standards. One of these that was widely used when studying reformulated fuel is called Tier 2 EEE. The Tier 2 EEE curve plotted here is from a tested sample used in the ACE Optimal Blend Study 2007. In other studies they use their own baseline. Shown below are distillation curves from some of the fuels from the "CRC-E67" study by Durbin, et al.
Fuel A was the baseline,
Fuel B wsa similar but with 5% eth
Fuel K was a baseline using MTBE laced fuel, 0% ethanol.
Fuel L was similar with MTBE but 10 % eth.
Not surprising, the fuels that evaporate more at lower temperatures tend to have a higher Reid Vapor Pressure.
RVP for:
Chevron 6.7
Fuel A 7.74
Fuel L 7.6
Tier 2 EEE 9.07