What happened to gasoline in the mid 1970s?
Octane rating is not directly related to the vaporization characteristics. For that look at the Reid Vapor Pressure (RVP) and the distillation curve.
The distillation curves below provide the general relationship of how much a fuel will evaporate at a given temperature.
In the graph I plotted a non-reformulated gasoline from 2007, and one from that time with 8% ethanol.
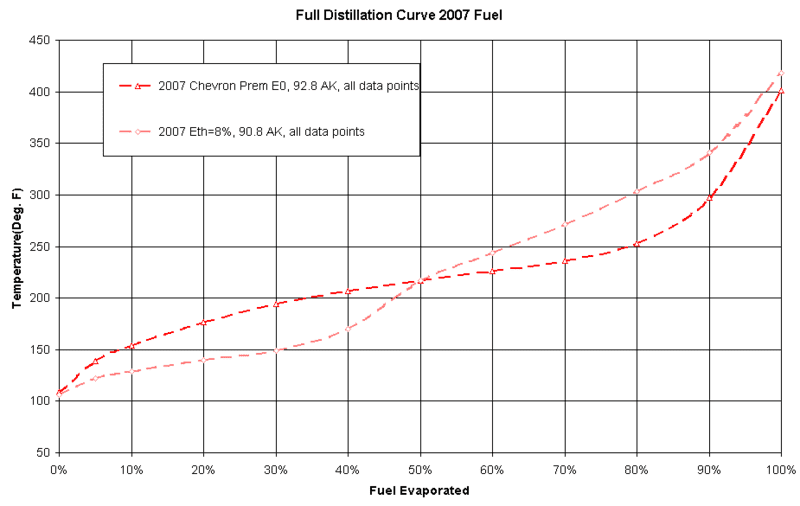
Read the graph like this:
Find a temperature on the left. Take 150* F. Follow the line across to the Chevron Premium, and then go straight down to the bottom axis. For 150*F, we see about 7% of this fuel evaporated. Now take the same 150* F and follow it to the E8 fuel. Looking down it shows 30% of the fuel has evaporated. Big Difference!
Also notice the shape of the E8. The light portion vaporizes easily, but then there is a jump to the mid range and completion. This can disrupt the flame front in a relative cold cylinder. Think of it like building a fire with lots of tinder and then skipping the small kindling.
Some other fuels plotted on the second graph in this post: Video of boiling point of fuel
As noted there, it seems that higher RVP correlateds with fuels that evaporate more volume at low termperatures.
The Feds allow higher 1. psi higher rvp with alcohol blends, but some states don't.
All retailers should now be selling summer fuel. Date of switch to summer-grade gasoline approaches - Today in Energy - U.S. Energy Information Administration (EIA)
Octane rating is not directly related to the vaporization characteristics. For that look at the Reid Vapor Pressure (RVP) and the distillation curve.
The distillation curves below provide the general relationship of how much a fuel will evaporate at a given temperature.
In the graph I plotted a non-reformulated gasoline from 2007, and one from that time with 8% ethanol.
Read the graph like this:
Find a temperature on the left. Take 150* F. Follow the line across to the Chevron Premium, and then go straight down to the bottom axis. For 150*F, we see about 7% of this fuel evaporated. Now take the same 150* F and follow it to the E8 fuel. Looking down it shows 30% of the fuel has evaporated. Big Difference!
Also notice the shape of the E8. The light portion vaporizes easily, but then there is a jump to the mid range and completion. This can disrupt the flame front in a relative cold cylinder. Think of it like building a fire with lots of tinder and then skipping the small kindling.
Some other fuels plotted on the second graph in this post: Video of boiling point of fuel
As noted there, it seems that higher RVP correlateds with fuels that evaporate more volume at low termperatures.
The Feds allow higher 1. psi higher rvp with alcohol blends, but some states don't.
All retailers should now be selling summer fuel. Date of switch to summer-grade gasoline approaches - Today in Energy - U.S. Energy Information Administration (EIA)