What in 'ell am I doing up this late??
And that is also how swamp coolers work. When you evaporate water from liquid to vapor, this is known as a "change of state."
This is known variously as "heat of vaporization", or if going the other way, "heat of condensation", etc
For example, if you raise the temerature of a pound (about a pint) of water from just above freezing to the boiling point under standard conditions (sea level atmospheric) that's 32F to 212F, or a change of 180 degrees F. For that one pound of water, that's therefore a change of 180 BTU in heat---which ain't all that much.
HOWEVER if you take that 1 pound of water and BOIL it down to the last drop gone, water has a "heat of vaporization" of 970 BTU per pound. THAT'S RIGHT!!! Nearly 1000 BTU for boiling off 1 pound of water!!
All substances which can exist in liquid and vapor form have similar properties, that is, much MORE heat is required to vaporize the liquid into vapor, and the SAME amount of heat is required to condense the vapor back to a liquid
THIS IS IN FACT the very basis of refrigeration systems. You cannot just "pump" the liquid or gas refrigerant around and around.
Med. temp, high pressure liquid is forced through a "control device", a capillary tube, an orifice, or thermal expansion valve. This huge change in pressure going from the liquid line, through the big drop in pressure entering the evaporator (which the compressor has lowered the pressure of) causes the liquid to "flash boil" which LOWERS it's temperature
(This is why air tools get COLDER as you use them instead of HOTTER--the air and the humidity in the air drops pressure tremendously as it exits the air tool, and drops the temperature of the air)
The evaporating liquid refrigerant (boiling) has now entered the evaporator and is ACCEPTING or ABSORBING heat from the hot air entering the evaporator fins pushed by the blower. This hot air exchanges heat into the boiling, evaporating refrigerant, and FURTHER boils off any remaining liquid, which of course REMOVES heat from the air, and out it comes from the supply side of the evap fins as ----nice cool air.
So now the boiled off refrigerant --which STILL is somewhat cool in a properly operating system, ----goes into the compressor inlet where it helps COOL the compressor valves and head, and in a home unit/ refrigerator, where the compressor is "hermetically sealed" it ALSO cools the drive motor.
The compressor compresses the refrigerant. An unwanted but unavoidable side effect of this compression is HEAT known, as, "heat of compression."
The hot, high pressure refrigerant now goes to the condenser, where relatively cool air -- pushed by vehicle speed, or a great big blower -- exchanges heat into the air and CONDENSES back to a liquid-----
and around and around we go.
This is why when you release CO2 from a cylinder, it forms "snow" -- the escaping CO2 cools rapidly through the orifice formed by the valve. It's why your hands get "too cold" if you handle thinner, cleaning solvents, gasoline, or any other volatile liquid. The RAPIDLY evaporating liquid produces a cooling effect
This "heat of condensation" or vaporization is ALSO why so called "90% plus" or "condensing" furnaces are able to obtain efficiencies of WELL over 90% AFUE.
What happens is this: The furnace burns propane or natural gas in a HOPEFULLY fairly efficient (80%) burner, which works much like older 70-80% AFUE furnaces in many cases.
THIS combustion produces LOTS of HOT water vapor AND superheated steam, which waltzes right out the vent and is wasted!!!
BUT in a "condensing" furnace, the first thing the circulating blower "sees" is the "secondary heat exchanger." AFTER the condensing furnace combusts the gas in what (can) amount(s) to a fairly standard burner, the hot, water vapor laden combustion products go through the "primary" heat exchanger, give up some of the heat in those gases, and then enter the SECONDARY heat exchanger.
In many furnaces, this secondary heat exchanger looks MUCH like an A/C evaporator, IE a finned heat exchanger. THE BLOWER, forcing cool "return" air from the conditioned space, REMOVES heat from the secondary exchanger, and this heat exchange----------------
CONDENSES the hot steam and water vapor out of the combustion gases. In many of these furnaces, often vented !!! with PVC!!! pipe, the vent gas temeratures are COOLER than the warm supply air leaving the plenum and going to the conditioned space!!!
Now this CONDENSED water simply drains off, rather than going up the vent!!!
(This is where the nearly 1000 BTU/ pound comes in --- for every POUND of water that drains out of a condensing furnace, that is NEARLY a thousand BTU that otherwise would have gone straight up the vent)
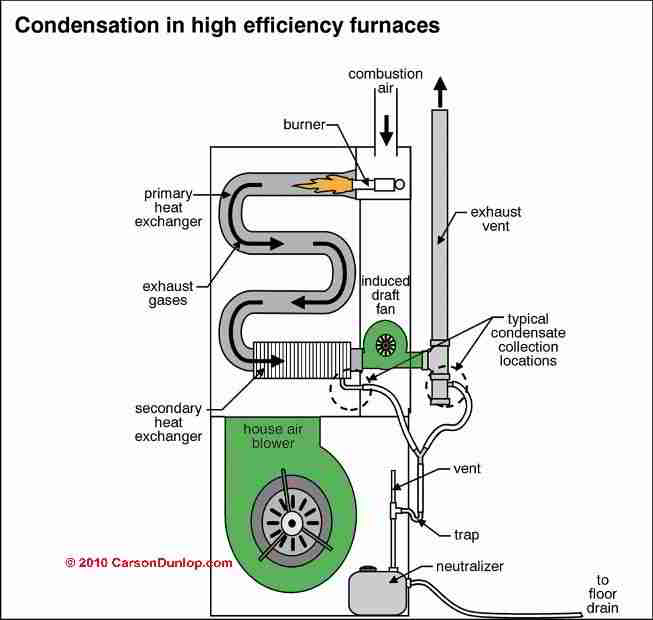
See below the generic representation of a condensing furnace. This one has the burner at the top, with an "induced draft" blower at the bottom. The induced draft blower encourages a draft through the system and out the vent, the burner fires into the top of the heat exchanger, and the hot gases make their way down through the primary, then the secondary heat exchanger.
Meanwhile the main circulating blower (at the bottom) forces "return" air from the house up through the two heat exchangers, removing heat from the heat exchangers, and causing the condensing effect.